by Piter Kehoma Boll
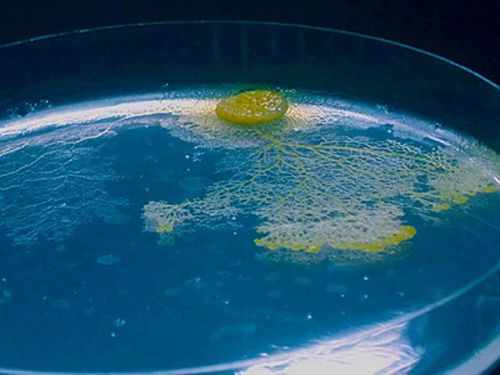
The slime mold Physarum polycephalum can “remember” where it has been even without a brain. Photo by Audrey Dussutour extracted from news.nationalgeographic.com
News
- DNA has a 521-year half-life, no DNA in dinosaur bones, guys. Get over it!
- Slime Has Memory but No Brain. Step by step we are discovering that other living creatures are not that stupid!
- Colored Honey Made by Candy-Eating French Bees. That’s bizarre! And it looks kinda disgusting too.
- New Genus of Ferns Named for Lady Gaga. That’s sooo disapointing!
- Scientists Say White Whale Mimics Human Speech. Kinda astonishing!
- Turtles Urinate via Their Mouths – A First. Things are getting a lil bit too bizarre now.
- New Sea Snake Species Found in… Museum. I bet there are lots of undescribed species in museums all over the world.
- Scientist Read Dreams. Now it’s possible to know what someone is dreaming!
- How to Eat Triceratops. Enjoy your meal!
- Vostok’s microbes elusive in first measurements of surface water. So by now it seems that Vostok is dead, Jim.
- “Penis Worm” Shakes Evolutionary Tree. When the mouth come after the anus, and it should have been the opposite.
- Were Neanderthals And Mordern Humans Birds of a Feather? It appears to be soo, literally with feathers!
Blog
- Pikaia: One of the Earliest Chordates. This is a creature everyone must know!
- Punk Moth, what a lovely larva!
- Miller, Urey and Venter, a nice post about what’s life and how it moves on.
- Tiny Tuco-tuco Teeth. Darwin’s view of this epic South-American rodent.
Art
- OneZoom Tree of Life Explorer. An amazing interactive tree of life where you can explore species through their position in the tree of life. Currently only mammals are available, but it’s worth taking a look!
Scientific Articles
- Deuterostomic Development in the Protostome Priapulus caudatus. So the protostome-deuterostome idea was an ilusion!
- When Less is More: Evolutionary Origins of the Affect Heuristic. Monkeys are as stupid as we are!
- There Is No News Like Bad News: Women Are More Remembering and Stress Reactive after Reading Real Negative News than Men. Bad news to you, girls…
- Ancient Origin of the Modern Deep-Sea Fauna. The abyssal zone may not have been aware of the mass extinctions up here.
- Ancient Ephemeroptera-Collembola Symbiosis Fossilized in Amber Predicts Contemporary Phoretic Associations. Those little hitchhiking bugs!
- Dolphins Can Maintain Vigilant Behavior through Echolocation for 15 Days without Interruption or Cognitive Impairment. When will Cetacea stop to surprise us?
- The Visual Perception of the Ant Myrmica ruginodis (Hymenoptera: Formicidae). Ants don’t see as bad as you think!
- The earlist known stem-tetrapod from the Lower Devonian of China. One more pal for the out-of-the-water novel!
- Feathered Non-Avian Dinosaurs from North America Provide Insight into Wing Origins. More and more we are discovering that non-avian dinosaurs are actually more avian that we thought!
- Slime mold uses and externalizes spatial “memory” to navigate in complex environments. Perception and memory far beyond animals.
- The social biology of domiciliary cockoraches: colony structure, kin recognition and collective decisions. Aren’t cockroaches in fact cute?
- Lethally Hot Temperatures During the Early Triassic Greenhouse. You won’t complain about the summer anymore after reading this.
- Neuroanatomy of Halobiotus crispae (Eutardigrada: Hypsibiidae): Tardigrade brain structure supports the clade panarthropoda. So water bears and bugs are indeed close relatives, it seems.
- Endosymbiotic calcifying bacteria: a new cue to the origin of calcification in Metazoa? Lynn Margulis is laughing in her grave.





