by Piter Kehoma Boll
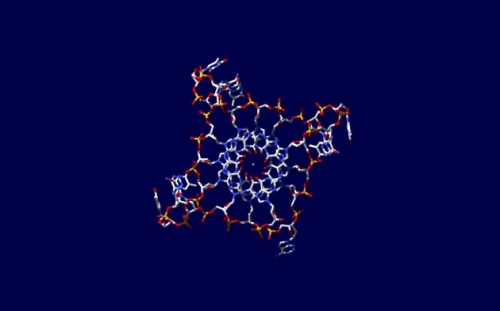
For the first time, the strange four-strand DNA has been found in cells! Picture by Jean-Paul Rodriguez, extracted from nature.com
News
- Zoologger: The first solar-powered vertebrate. A charming salamander has also made some deals with algae!
- Four-strand DNA structure found in cells. DNA four-strand form was already known in lab, but for the first time it was found in cells.
- Letters of Alfred Russel Wallace Go Online. Because we cannot forget the co-author of the theory of natural selection.
- Eat Less by Altering Your Food Memories. You may get satiated just by remembering food. How cool is that, huh?
- Critically Endangered Mexican Gray Wolf Released into Arizona Wild. We wish good look to our fellow!
- Dog’s dinner was key to domestication. A change in wolves’ digestion seems to have originated our friends.
- Synthetic double-helix faithfully stores Shakespeare’s sonnets. It would be interesting to transcript and translate such a DNA strand.
- Almost Extinct Brazilian Bird Observed in Nest for the First Time. The Stresemann’s bristlefront, Merulaxis stresemanni has been recorded in video!
- Dung Beetles Use Milky Way to Guid Movement. This is the first species to be known to do so!
Blog
- Will Grey Parrots Share? Personality is a complex feature even in parrots!
- Hiding your research behind a paywall is immoral, by Mike Taylor. Excellent article!
- Can Explanations Make Learning More Difficult? An interesting and revolutionary way to think.
- Gut Microbes Contribute to Mysterious Malnutrition. It’s not only our own DNA that defines our body.
- Healing the Brain with Snail Veenom. Conotoxins seem to be a pot of gold for medicines!
- Corridors Critical for India’s Big Cats. Habitat fragmentation is a major issue for endangered species and corridors uniting this fragments are essential for their survival.
- The strangest cancer in the world. Tasmanian devils are threatened by a scary contagious cancer. 😦
- Secret of the Dinosaur Sexes Locked in Bone. Medullary Bone may hold the key.
- Tweet to Learn. You can indeed learn a lot by using twitter! By the way, Earthling Nature is on Twitter too! 😉
- Echidna ejaculation is a little one-sided. Take a look at the most bizarre penis in the animal kingdom!
- How to Cross the Deep Sea: Stepping Stones of Mollusk Poop. The mysterious deep sea life is indeed very connected to the surface.
Art & Books
- The *New* Big Golden Book of Dinosaurs. Take a look and compare how our view of dinosaurs has changed! You can find it at Amazon, here!
- Kangaroos, Australia, by Gordon Fellows. Beautiful shot!
Scientific Articles
- Intracapsular algae provide fixed carbon to developing embryos of the salamander Ambystoma maculatum. The article about the “photosynthetic” salamander.
- Quantitative visualization of DNA G-quadruplex structures in human cells. The paper about the four-strand DNA.
- The more polluted the environment, the more important biodiversity is for food web stability. Unfortunately it seems to work against the flow.
- A New Azhdarchid Pterosaur from the Late Cretaceoous of the Transylvanian Basin, Romania: Implications for Azhdarchid Diversity and Distribution. Our new fossil guy is called Eurazhdarcho langendorfensis.
- Reduced plumage and flight ability of a new Jurassic paravian theropod from China. Eosinopteryx’s plumage indicates that deinonychosaurs occupied a wider range of niches than previously thought.
- Gender identification of the Mesozoic bird Confuciusornis sanctus. Because some small details can show you who is a boy and who is a girl.
- Evolutionary Applications, with a special issue relating cancer and evolution.
- Hailstones: A Window into the Microbial and Chemical Inventory of a Storm Cloud. There are more bacteria living inside the clouds than you imagine.
- Consumption with Lagre Sip Sizes Increases Food Intake and Leads to Underestimation of the Amount Consumed. More tips for losing weight!
- The First Metriorhynchid Crocodylomorph from the Middle Jurassic of Spain, with Implications of Evolution of the Subclade Rhacheosaurini. This month has plenty of interesting fossil findings!