by Piter Kehoma Boll
Parasites are special types of organisms that live on or inside other lifeforms, slowly feeding on them but usually not killing them, just reducing their fitness to some degree. This is a much more discrete way to survive than killing or biting entire parts off, as predators (both carnivores and herbivores) do. However, different from these creatures, parasites are often regarded as unpleasant and disgusting. Yet parasitism is the most common way to get food in nature.
When I introduced the rhinoceros tick in a recent Friday Fellow, I mentioned the dilemma caused by it. Since the rhinoceros tick is a parasite of rhinoceroses, and rhinoceroses are threatened with extinction, a common practice to improve the reproductive fitness of rhinos is removing their ticks, but this may end up leading the rhinoceros tick to extinction.
This actually happened already with other parasites, such as the louse Coleocephalum californici, which was an exclusive parasite of the California condor Gymnogyps californianus. In order to save the condor, a common practice among veterinarians working with conservationists was to delouse the birds and, as a result, this louse is now extinct. The harm that the louse caused to the condor was so little, though, that its extinction was not at all necessary, being nothing more than a case of negligence and lack of empathy for a small and non-charismatic species.

The louse Rallicola (Aptericola) pilgrimi has also vanished forever during the conservation campaigns to save its host, the little spotted kiwi, Apteryx owenii, in another failed work.


Another group of parasites that is facing extinction are fleas. The species Xenopsylla nesiotes was endemic to the Christmas Island together with its host, the Christmas Island rat, Rattus macleari. The introduction of the black rat, Rattus rattus, in the island led to a quick decline in the population of the Christmas Island rat, which went extinct at the beginning of the 20th century and, of course, the flea went extinct with it. The flea Acanthopsylla saphes has likely become extinct as well. It was a parasite of the eastern quoll, Dasyurus viverrinus, in mainland Australia. The eastern quoll today is only found in Tasmania, as the mainland Australia’s population went extinct in the mid-20th century. However, the flea was never found in the Tasmanian populations, so it is likely that it died away in mainland Australia together with the local population of its host.
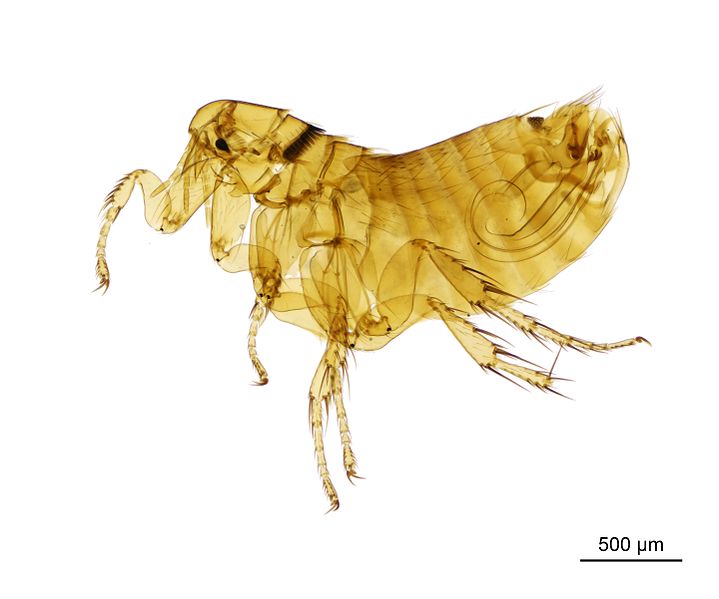
But things have been changing lately and fortunately the view on parasites is improving. A recent assessment was made on the population of another flea, the Manx shearwater flea, Ceratophyllus (Emmareus) fionnus. This flea is host-specific, being found only on the Manx shearwater Puffinus puffinus. Although the Manx shearwater is not at all a threatened species and has many colonies along the North Atlantic coast, the flea is endemic to the Isle of Rùm, a small island off the west coast of Scotland. Due to the small population of its host in this island, the flea has ben evaluated as vulnerable. If the Manx shearwater population in the Island were stable, things would be fine but, as you may have guessed already, things are not fine. Just like it happened in Christmas Island, the black rat was also introduced in the Isle of Rúm and has become a predator of the Manx shearwater, attacking its nests.

Some ideas have been suggested to protect the flea from extinction. One of them is to eradicate the black rat from the Isle or at least manage its population near the Manx shearwater colonies. Another proposal is to translocate some fleas to another island to create additional populations in other Manx shearwater colonies.
But why bother protecting parasites? Well, there are plenty of reasons. First, they comprise a huge part of the planet’s biodiversity and their loss would have a strong impact on any ecosystem. Second, they are an essential part of their host’s evolutionary history and are, therefore, promoters of diversity by natural selection. Removing the parasites from a host would eventually decrease its genetic variability and let it more vulnerable to other new parasites. Due to their coevolution with the host, parasites are also a valuable source of knowledge about the host’s ecology and evolutionary history, helping us know their population dynamics. We can even find ways to deal with our own parasites by studying the parasites of other species, and parasites are certainly something that humans managed to collect in large numbers while spreading across the globe.
Parasites may be annoying but they are necessary. They may seem to weaken their host at first but, in the long run, what doesn’t kill you makes you stronger.
– – –
– – –
References:
Kirst ML (2012) The power and plight of the parasite. High Country News. Available at < https://www.hcn.org/blogs/goat/the-power-and-plight-of-the-parasite >. Access on 3 November 2019.
Kwak ML (2018) Australia’s vanishing fleas (Insecta: Siphonaptera): a case study in methods for the assessment and conservation of threatened flea species. Journal of Insect Conservation 22(3–4): 545–550. doi: 10.1007/s10841-018-0083-7
Kwak ML, Heath ACG, Palma RL (2019) Saving the Manx Shearwater Flea Ceratophyllus (Emmareus) fionnus (Insecta: Siphonaptera): The Road to Developing a Recovery Plan for a Threatened Ectoparasite. Acta Parasitologica. doi: 10.2478/s11686-019-00119-8
Rózsa L, Vas Z (2015) Co-extinct and critically co-endangered species of parasitic lice, and conservation-induced extinction: should lice be reintroduced to their hosts? Oryx 49(1): 107–110. doi: 10.1017/S0030605313000628
– – –
* This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
** This work is licensed under a Creative Commons Attribution-ShareAlike 3.0 Unported License.
This work is licensed under a Creative Commons Attribution-ShareAlike 3.0 Unported License.
*** This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License.








