by Piter Kehoma Boll
The complexity of ecosystems is sustained by a variety of relationships that different species have with each other and that are often adapted to the environment in which they live. Although we usually think of relationships based on conflicts, such as predation, parasitism, and competition, beneficial relationships are almost as important and common, especially when we think of flowering plants, as many plant species rely on animals to pollinate them and disperse their seeds.
The different ways through which plants are pollinated are called pollination syndromes and include anemophily (pollination by wind), melittophily (by bees), phalenophily (by moths), sphingophily (by hawk moths), psychophily (by butterflies), myophily (by flies), cantharophily (by beetles), chiropterophily (by bats) and ornithophily (by birds), and there are generalist plants as well, whose flowers can be pollinated by several different animals. Now considering the way plants have their seeds dispersed, the classification is usually into only three categories: zoochory (by animals), anemochory (by wind), and autochory (by the plant itself, because why wait for animals or the wind? Ain’t nobody got time for that!).

We often think of bees and butterflies as the most common pollinators. Indeed bees are by far the most common and important, but actually very few plants rely exclusively on butterflies for pollination. Flies, beetles, moths, and even birds and bats often pollinate more plant species in a given ecosystem. Regardless of the ecosystem being a dense forest, an open grassland, or a shrubby savanna, bees are always the ones doing the job for most plant species.
Now regarding seed dispersal, the configuration of the ecosystem is much more important and causes drastic changes in the frequency of dispersal syndromes. In open areas such as grasslands and savannas, anemochory is often considered to be the predominant dispersal syndrome. In forests, however, zoochory would dominate, as there is not enough wind to blow seeds around.
When we survey the dispersal syndromes in forests, we find that most plant species have, indeed, their seeds dispersed by animals. However, a survey in grasslands and savannas can show results that look puzzling at first. Sometimes all three dispersal syndromes occur in the same proportion and sometimes lots of species continue to be dispersed by animals, while the wind is important only to a few. Were we wrong in our predictions then? Not necessarily.
One problem is that most studies, almost all actually, only compare pollination and dispersal syndromes by the number of species in that area. However, plant species are not evenly distributed in the environment. Some species have lots of individuals, being dominant in their ecosystems, while others occur in a much smaller number. Does the proportion of dispersal syndromes remain the same if we consider the number of individuals and not species? Not necessarily.
A recent study evaluated the pollination and dispersal syndromes of plants in an area of the Brazilian Cerrado biome, more specifically an area of Cerrado Rupestre (one of the less known Cerrado physiognomies). The researchers not only considered the distribution of the syndromes according to the number of species but also according to the number of individuals. Most plant species were pollinated by bees, as expected, and most individuals were pollinated by bees as well. However, while most species had their seeds dispersed by animals, most individuals had their seeds dispersed by wind. This means that, although most species rely on animals to disperse seeds, they tend to occur in a lower density, with fewer individuals per area. On the other hand, wind-dispersed species have a very high density, so most individuals in an area belong to them.
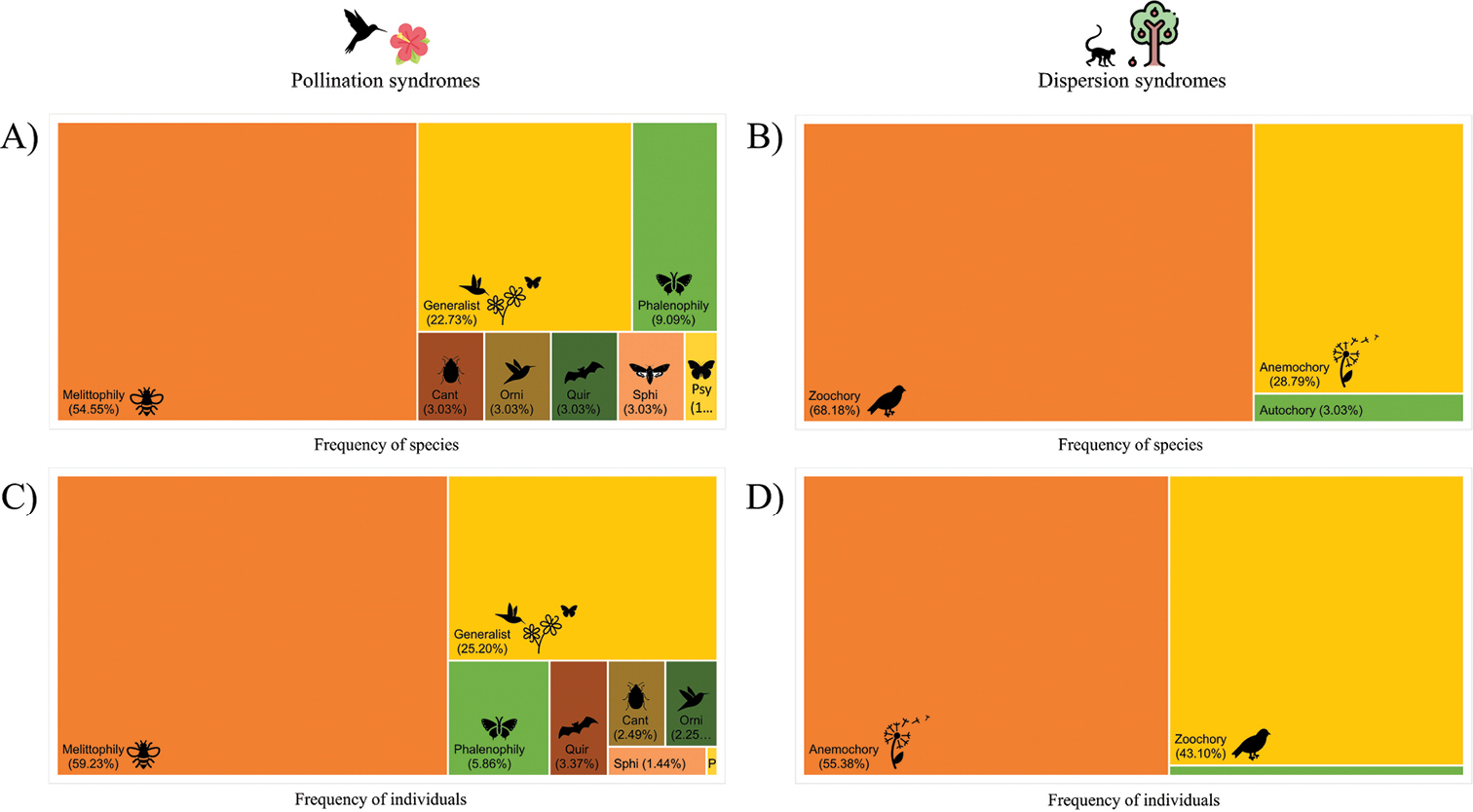
When we consider the distribution of dispersal syndromes only according to species, the results seem to contradict what is expected for a savanna, but looking at it from the perspective of individuals makes it clear that the pattern follows the predictions.
Being aware of this is important for several reasons, especially to allow adequate management programs to protect such areas. The stability of an ecosystem does not depend solely on the species richness but also on the abundance of each species. By analyzing the distribution of dispersal syndromes from both perspectives, we can see that the wind is the main disperser for this ecosystem as a whole, but animals are still important dispersers to keep the species richness high and, in turn, a high richness of plant species is important to sustain the animal species. This makes our understanding of the whole system very different from what we would know from data on species alone. Now let’s hope future studies will start to address this issue from both perspectives as well.
– – –
– – –
References:
Kuhlmann, M., & Ribeiro, J. F. (2016). Evolution of seed dispersal in the Cerrado biome: ecological and phylogenetic considerations. Acta Botanica Brasilica, 30, 271-282. https://doi.org/10.1590/0102-33062015abb0331
Pereira, C. C., Arruda, D. M., Soares, F. D. F. S., & Fonseca, R. S. (2022). The importance of pollination and dispersal syndromes for the conservation of Cerrado Rupestre fragments on ironstone outcrops immersed in an agricultural landscape. Neotropical Biology and Conservation, 17(1), 87-102. https://doi.org/10.3897/neotropical.17.e79247
– – –
* This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.