by Piter Kehoma Boll
Here is a list of species described this month. It certainly does not include all described species. You can see the list of Journals used in the survey of new species here.
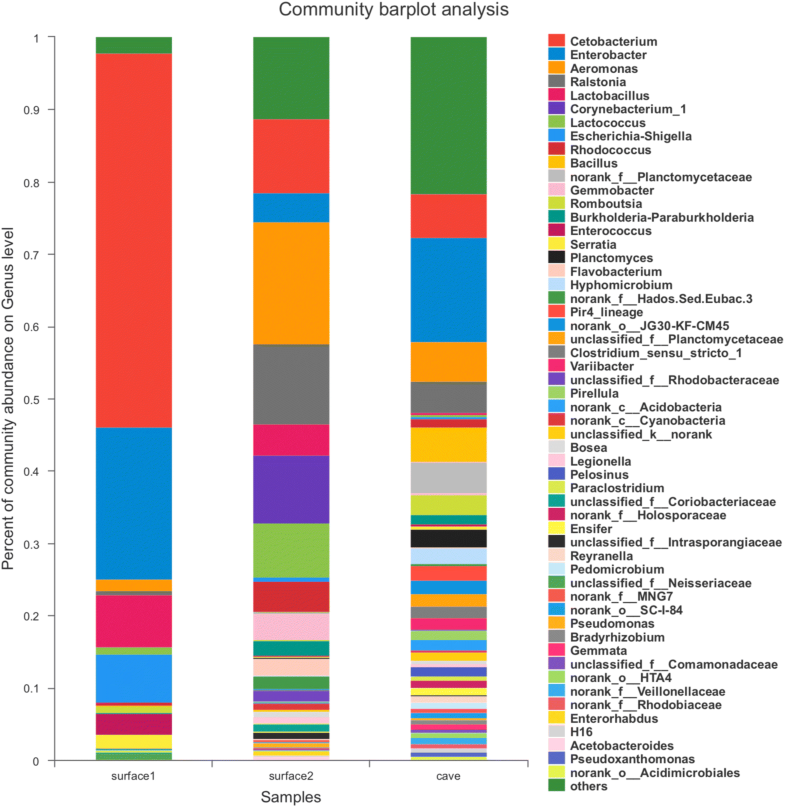
Bacteria
- 8 new actinobacteria: Haloactinobacterium glacieicola; Nocardioides yefusunii; Nocardioides guangzhouensis; Nocardioides vastitatis; Antribacter gilvus; Rhodoluna limnophila; Microlunatus speluncae; Nocardia panacis;
- 6 new bacteroids: Flavobacterium ranwuense; Flavobacterium cellulosilyticum; Mucilaginibacter gilvus; Sphingobacterium puteale; Bacteroides faecalis; Chryseobacterium candidae;
- 8 new firmicutes: Paenibacillus xylanivorans; Paenibacillus thalictri; Paenibacillus pinistramenti; Schaedlerella arabinosiphila; Biomaibacter acetigenes; Bacillus cabrialesii; Bacillus lacisalsi; Lactobacillus xujianguonis;
- 15 new proteobacteria: Pseudomonas tructae; Methylobacterium oryzihabitans; Methylobacterium durans; Bradyrhizobium frederickii; Leucothrix sargassi; Salaquimonas pukyongi; Halobacteriovorax vibrionivorans; Rhodoferax bucti; Rhodoligotrophos defluvii; Lampropedia aestuarii; Halomonas radicis; Marinomonas agarivorans; Lichenihabitans psoromatis; Entomomonas moraniae; Rhizobium glycinendophyticum; Lacisediminimonas profundi;
Archaeans
- 1 new species: Halobellus captivus;
SARs
- 2 new ciliates: Chlamydodon pararoseus; Oxytricha seokmoensis;
- 1 new oomycete: Aphanomyces brasiliensis;
- 1 new diatom: Nediomorpha gusevii;

Plants
- 1 new rhodophyte: Sheathia matouensis;
- 6 new pteridophytes: Hymenasplenium guineense, H. kenyense, H. sabahense, H. wusugongii; Bolbitis lianhuachihensis; Asplenium simaoense;
- 57 new angiosperms: Neea itanhaensis; Henckelia collegii-sancti-thomasii; Eugenia lasiothecia; Dendrobium nagataksaka; Stachytarpheta sobraliana; Ceanothus fernandezii; Bulbophyllum chelicerum; Oenothera resicum; Eriocaulon rayatianum; Paramongaia multiflora, Rauhia albescens; Telosma thailandica; Nabalus muliensis; Bunium sivasicum; Scoparia pentapetala; Croton seccoi; Vicia brulloi; Saxifraga shennongii; Gustavia esmeraldana, G. gracieae; Peliosanthes revoluta; Amomum erythranthum, Amomum ampliflorum; Chusquea gamarrae, C. intipaqariy; Ternstroemia acajetensis; Crocus keltepensis; Memecylon kosiense, M. soutpansbergense, M. australissimum; Megalachne sp.; Codonoboea norakhirrudiniana, C. rheophytica, C. sallehuddiniana; Isoetes dubsii, I. santacruzensis; Oreocharis tetrapterus; Clinanthus inflatus, Ismene parviflora; Swertia hongquanii; Primulina serrulata; Rhamnella brachycarpa; Aulonemiella laegaardii; Inga kursarii; Rhynchospora chapadensis, Rhynchospora harleyi; Erythroxylum niziae; Fritzschia cordifolia; Serjania rosalindae, Serjania crucensis; Waltheria saundersiae; Tarenaya longicarpa; Varronia xinguana; Justicia birae, Justicia distichophylla , and Justicia mcdadeana; Oxylobus coyulensis;


Amoebozoans
- 1 new mycetozoan: Echinostelium microsporum;
- 1 new discosean: Vannella samoroda;
Fungi
- 1 new mucoromycete: Gongronella sichuanensis;
- 15 new ascomycetes: Murispora aquatica; Cordyceps qingchengensis; Tremateia murispora; Neopestalotiopsis hadrolaeliae; Thyrostroma ephedricola; Colletotrichum artocarpicola; Seiridium chinense; Paraisaria orthopterorum, P. phuwiangensis, P. yodhathaii; Beauveria peruviensis; Simplicillium cicadellidae, S. formicidae, S. lepidopterorum; Metarhizium brachyspermum;
- 15 new basidiomycetes: Heimioporus sinensis; Marasmiellus griseobrunneus; Lactarius brunneocinnamomeus; Infundibulicybe kotanensis; Protomerulius commotus, Protomerulius hebes, Protomerulius madidus, Protomerulius pertusus, Psilochaete multifora; Inocybe botaurina, I. bombina, I. undinea; Clavulina mahiscolorata, C. parvispora; Clitopilus lampangensis;

Sponges
- 5 new hexactinellids: Calyptorete falkorae, Farrea ritchieae, F. hieroglyphica, Amphidiscella hosiei, Rhabdocalyptus gomezi;
- 7 new demosponges: Erylus imperator, Geodia arma; Clathria (Axosuberites) aurantia, Clathria (Axosuberites) hillenburgi; Antho (Plocamia) sarasiri; Tsitsikamma michaeli, Tsitsikamma nguni;

Cnidarians
- 4 new anthozoans: Swiftia sahlingi; Ceriantheomorphe adelita; Porites farasani, Porites hadramauti;
Flatworms
- 2 new planarians: Cratera obsidiana, Luteostriata subtilis;
- 2 new monogeneans: Gyrodactylus decemmaculati, Gyrodactylus breviradix;
- 4 new trematodes: Asaccotrema vietnamiense; Galactosomum otepotiense; Brachylaima lignieuhadrae; Notocotylus primulus;
Bryozoans
- 1 new species: Pleurocodonellina jeparaensis;
Annelids
- 33 new polychaetes: Terebellides bonifi, T. ceneresi, T. europaea, T. gentili, T. gralli, T. lilasae, T. parapari, T. resomari, Trichobranchus demontaudouini; Aphelochaeta caribbeanensis, C. angusticrista, C. convexacapa, C. microbidentata, C. parapicula, C. parvinasa, C. quadrata, Chaetozone dossena, Kirkegaardia filiformis, K. panamaensis, K. playita, Dodecaceria alphahelixae, D. dibranchiata; Hyalopale leslieae, H. zerofskii, H. sapphiriglancyorum, H. angeliensis, H. furfuricula; Marphysa iloiloensis; Struwela camposi; Notodasus celebensis, N. chinensis;
- 4 new clitellates: Amynthas demptus, Amynthas lacustris, Metaphire reclusa; Petroscolex centenarius;
Mollusks
- 1 new gastropod: Satsuma guandi;
Nematodes
- 1 new species: Labrys khuzestanensis;
Tardigrades
- 3 new species: Cryoconicus antiarktos, Ramazzottius sabatiniae; Macrobiotus caelestis;
Arachnids
- 3 new mites: Leptus (Leptus) haitlingeri; Diptilomiopus indogangeticus, Diptilomiopus mohanasundarami;
- 7 new spiders: Otacilia fansipan; Apopyllus kanguery; Extraordinarius andrematosi, E. brucedickinsoni, E. klausmeinei, E. rickalleni; Teyl heuretes;
- 2 new pseudoscorpions: Neochelanops unsaac; Spelaeochernes popeye;
- 5 new harvestmen: Panzosus comayagua, Panzosus cusuco, Panzosus giribeti, Panzosus marginalis, Panzosus roeweri;
Myriapods
- 2 new diplopods: Taiyutyla caseophila, Opiona catorycha;
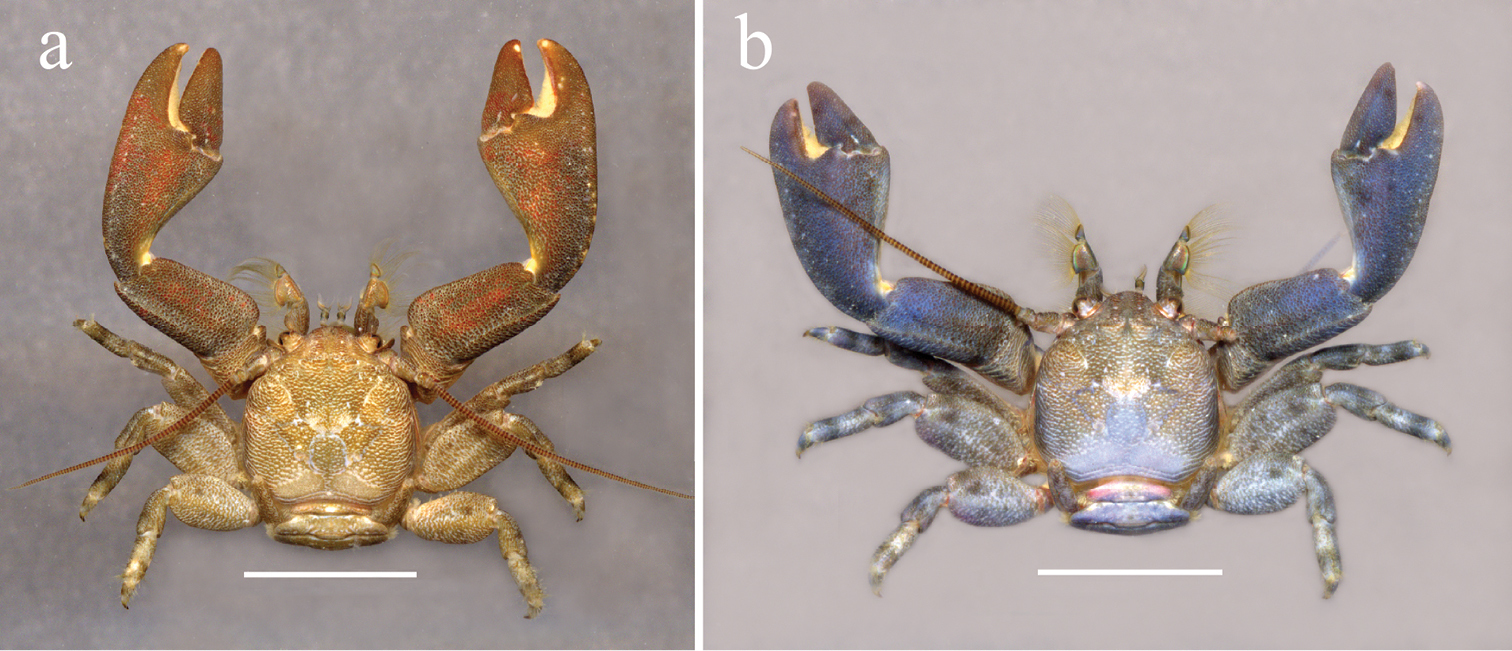

Crustaceans
- 2 new copepods: Monstrilla chetumalensis; Dermoergasilus cichlidus;
- 1 new ascothoracid: Synagoga arabesque;
- 1 new tanaid: Tanaella quintanai;
- 1 new amphipod: Polycheria spongoteras;
- 3 new isopods: Alpioniscus busljetai; Xiphoarcturus kussakini, Xiphoarcturus carinatus;
- 9 new decapods: Cinetorhynchus gabonensis; Macrobrachium chainatense; Strengeriana quindiensis; Pugettia ferox; Arcithelphusa tumpikkai; Petrolisthes virgilius; Caridina enbilului, Caridina enkii, Caridina shahrazadae;

Hexapods
- 1 new proturan: Acerentulus bulgaricus;
- 3 new collembolans: Homidia chroma, Homidia leniseta; Willemia panamaensis;
- 4 new ephemeropterans: Rhoenanthus (Rhoenanthus) tungaiensis; Deanophlebia radsjoshi; Behningia nujiangensis; Simothraulopsis primus;
- 4 new odonates: Mecistogaster kesselringi , M. mielkei, M. nordestina; Heteragrion denisye;
- 30 new orthopterans: Saga hakkarica; Afroanthracites guttatus, A. maculatus, A. magamba, A. pommeri, A. lineatus, A. inopinatus, Afroagraecia spp., Dendrobia plagata; Glaphyrosoma stephanosoltis; Kefalia grafika, K. laeta, K. omorfa, Sentia communa, Melidia adfinia, Horatosphaga laticerca, Horatosphaga scalata, Peronura wottae, Tenerasphaga mpwapwae; Gorochovius furvus; Pentacentrus dulongjiangensis; Tachycines (Gymnaeta) lalinus; Polionemobius marblus, Ornebius panda; Scaria rafaeli, S. jonasi, S. granti;
- 4 new plecopterans: Andiperla morenensis; Kamimuria peppapiggia; Leuctra khachapuri; Capnia khingana;
- 2 new thysanopterans: Merothrips meridionalis; Odontothrips moritzi;
- 29 new hemipterans: Macropsis tincta, M. viridofulgida, M. nikippa, M. antibia; Sinotympana caobangensis; Dwightla lancea; Ulopsina bimaculata; Reticuluma lageniformia, R. hamata; Nepiomistus eximius; Changbaninus furcatus, Changbaninus pleiospicules; Baya lata, Zinga longa, Pettya tenuis; Asialeyrodes nicobarica; Mycetocylapus alexeyi, Punctifulvius austellus, Punctifulvius aquilonius, Rhinocylapoides valentinae; Psylla frodobagginsi; Lembakaria saintemariae, Lembakaria mikeae; Anisoedessa proctocarinata, A. bispinosa, A. proctolabiata, A. flavomaculata, A. calodorsata, A. ypsilonlineata;
- 130 new coleopterans: Proterhinus tauai; Pyrrhalta carolusi, P. kaboureki, P. kambaitiensis, P. lucka, P. schillhammeri, P. tsoui; Lacvietina nabanhensis; Trizogeniates beckeri, T. curvatus, T. eliskae, T. hallensorum, T. spatulatus, T. vazdemelloi, T. zuzanae; Xanthonia hirsuta, X. marquai, X. nitida, X. parva, X. picturata, X. querci, X. texana; Qianaphaenops (Sanwangius) rowselli; Marcepania princesa; Eucinetus bicolor, Eucinetus bicolorellus, Eucinetus brindabellae, Eucinetus dorrigo, Eucinetus limitaris, Eucinetus lorien, Eucinetus minutus, Eucinetus nebulosus, Eucinetus protibialis, Eucinetus similis, Eucinetus tasmaniae, Eucinetus tropicus, Noteucinetus ornatus, Noteucinetus victoriae; Ascopus girardi; Eustra yinggelingensis; Versicorpus daures; Obereoides peruviensis, Morvanica demezi; Mawenzhena koreana; Hybalus bellmanni; Lemula (s. str.) aequipilosa, L. (s. str.) nitidiuscula, L. (s. str.) zhani, L. (s. str.) jinfoshana, L. (s. str.) formosana, L. (s. str.) simillima, L. (s. str.) holzschuhi, L. (s. str.) shaanxiensis, L. (Pseudolemula) tichyi; Ischnomera sp.; Pygopleurus brullei; Sarmydus nicobarensis; Paraneseuthia zanetae; Phoberus ntlenyanae; Cyanopenthe granulata, C. hirtiscutellara; Cylindera ooa, Cylindera autumnalis; Solariola angelinii, Solariola benellii, Solariola bucolorum, Solariola cajetanibelloi, Solariola calida, Solariola comata, Solariola diottii, Solariola fancelloi, Solariola forbixi, Solariola gratiensis, Solariola hyblensis, Solariola ientilei, Solariola margaritae, Solariola mariaeclarae, Solariola mariaesilvanae, Solariola mariaetheresiae, Solariola melonii, Solariola nemoralis, Solariola normanna, Solariola obsoleta, Solariola pacei, Solariola paulimagrinii, Solariola pentaphyllica, Solariola petriolii, Solariola poggii, Solariola raphaelis, Solariola rosae, Solariola sabellai, Solariola saccoi, Solariola sbordonii, Solariola selinusia, Solariola tedeschii, Solariola venusta, Solariola zoiai; Coelostoma jaculum, C. phototropicum; Bomansius cheesmanae; Pentilia bernadette, P. chelsea, P. dianna, P. elena, P. ernestine, P. estelle, P. kari, P. jasmine, P. jody, P. kendra. P. krystal, P. lora, P. mable, P. muriel, P. nichole, P. nadine. P. paulette, P. rachael, P. sadie and P. traci; Charaea boukali, Ch. nilgiriensis, Ch. sahyadrica; Trichocircus decoris, T. antennatus, T. miser; Holocanthon giosilvai; Alphasida (Alphasida) perezverai;
- 2 new neuropterans: Neoconis szirakii, Coniopteryx (Scotoconiopteryx) josephus;
- 38 new hymenopterans: Undabracon binduae; Neohybrizon mutus; Casinaria camura, C. scalaris, Venturia aquila; Coelinius carmenae, Coelinius danielae; Masona popeye; Hylophasma luica; Propristocera seediq; Gastrodynerus guatemalensis, G. barretti, G. aimara, G. yungaensis; Bracon planitibiae; Homalictus atritergus, H. concavus, H. groomi, H. kaicolo, H. nadarivatu, H. ostridorsum, H. taveuni, H. terminalis, H. tuiwawae; Oodera arabica, O. omanensis, O. rapuzzii, O. similis; Metapone murphyi; Dioxybracon maridadi, D. aurumoram, D. vannoorti; Borgatomelissa flavimaura; Caupolicana rupestris; Temnothorax almeqeri; Stenocorse atlanticus, S. maesoi, S. pacificus, S. rosabricenae, S. sudamericanus;
- 3 new mecopterans: Kohlsia misantlensis; Panorpa jinhuaensis, Panorpa menqiuleii;
- 59 new dipterans: Chrysomydas phoenix; Pagastia (P.) subletteorum; Trichocoelina absidata, T. aemula, T. biplex, T. dicksoni, T. dispansa, T. dividua, T. hians, T. imitator, T. incrassata, T. ithyspina, T. jukkai, T. magnifica, T. nefrens, T. obesula, T. oricillifera, T. planilobata, T. quintula, T. semisphaera, T. semusta, T. tecta; Rachicerus carrerai; Paraleucopis auripes, P. bispinosa, P. boharti, P. nigra, P. paraboydensis, P. saguaro; Rhamphomyia (Eorhamphomyia) shewelli, R. (Pararhamphomyia) frigida, R. (Pararhamphomyia) lymaniana, R. (Pararhamphomyia) petervajdai, R. (Pararhamphomyia) septentrionalis; Epiphragma (Epiphragma) acuminatum, E. (E.) henanensis, E. (E.) longitubum; Zeugodacus madhupuri; Phortica allomega, P. archikappa, P. dianzangensis, P. imbacilia, P. liukuni, P. tibeta, P. xianfui; Anasimyia diffusa, Anasimyia matutina, Brachyopa caesariata, Brachyopa cummingi, Hammerschmidtia sedmani, Microdon (Microdon) scauros, Mixogaster fattigi, Neoascia guttata, Orthonevra feei, Psilota klymkoi, Trichopsomyia litoralis; Lipurometriocnemus spp.;
- 44 new lepidopterans: Neadeloides nubilus; Cyana (Cyabarda) nambi, C. (Idiovulpecula) lowa, C. (Idiovulpecula) foya; Polyphena chongzuoensis; Barsine wangi; Nosphistica abunda, N. apiculata, N. subrectangula, N. elliptica, N. fusoidea, N. grandiunca, N. albimaculata; Tumicla elephantina, T. mbeghai, T. admiranda, T. smithi; Eupithecia nemrutica; Lissochlora klausi; Cidariplura nanling, C. luding, C. hbun; Barsine victoria, B. kanchenjunga, B. dejeani, B. thagyamin, B. hreblayi, B. siberuta; Promalactis curviprocessa, P. digitiuncata, P. equisaccula, P. circimacularis, P. foliuncata, P. spinivalvaris, P. tenuiclavata; Anacochylidia maderana, Pogospinia floridana, Monoceratuncus lantana, Aethes triassumenta; Drasteria pseudopicta; Tarmia greeneyi; Moca austrasinensis; Metzneria freidbergi, Scrobipalpa aravensis;

Tunicates
- 1 new species: Botrylloides conchyliatus;
Actinopterygians
- 5 new siluriforms: Cetopsis aspis; Pimelodella yaharo; Batrochoglanis castaneus; Baryancistrus micropunctatus, Baryancistrus hadrostomus;
- 4 new cypriniforms: Paraschistura makranensis; Pseudophoxinus cilicicus; Garra roseae; Enteromius thespesios;
- 1 new gadiform: Physiculus cirm;
- 2 new perciforms: Centropomus irae; Epinephelus fuscomarginatus;
Amphibians
- 1 new anuran: Microhyla eos;

Reptiles
- 11 new squamates: Cyrtodactylus dayangbuntingensis; Cyrtodactylus manos; Cordylus phonolithos; Hebius sangzhiensis; Saurodactylus splendidus, S. harrisii, S. slimanii, S. elmoudenii; Achalinus yunkaiensis; Lycodon pictus; Liopeltis pallidonuchalis;
- 1 new crocodile: Crocodylus halli;

Mammals
- 1 new rodent: Chaetodipus durangae;
– – –
* This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.