by Piter Kehoma Boll
Tiny and tough, our newest Friday Fellow can be found hidden among the moss throughout most of the planet, and perhaps even beyond it, for if there is a species to which space is a piece of cake, that species is the common water bear Milnesium tardigradum.

A Scaning Electron Microscope (SEM) image of a specimen of the common water bear in its active state. Photo extracted from Schokraie et al. (2012).*
You may have already heard of tardigrades or water bears, tiny chubby animals that are able to withstand the harsher conditions, such as intense desiccation, radiation and even the vacuum of outer space. Most of the data regarding the toughness of these organisms comes from the common water bear, the most widespread species of the phylum Tardigrada.
Measuring up to 0.7 mm in length, the common water bear has eight legs with claws on their end and is considered a predator, feeding on a variety of other small organisms, including algae, rotifers and nematodes. It has a worldwide distribution and is commonly found living on moss, such as the cosmopolitan silvergreen moss already presented here.
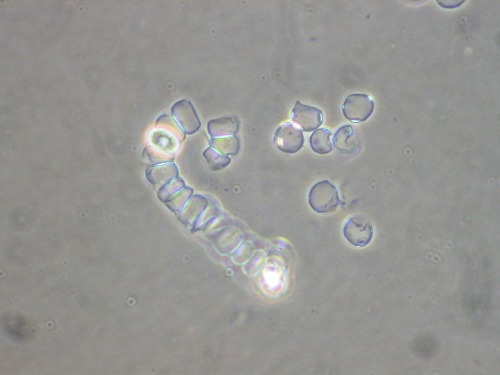
As members of the supergroup Ecdysozoa (which also includes arthropods and roundworms), tardigrades undergo ecdysis, also commonly known as molting, a process through which they shed their exoskeleton. In the common water bear, females always lay eggs around the time of molting. Before leaving the old exoskeleton, the females lay the clutch of eggs, which may vary from 1 to 12 eggs, between the old and the new exoskeleton and usually remain inside the old exoskeleton several hours after laying the eggs. When they finally leave, the eggs remain inside the shed skin, which perhaps helps them to be more protected from danger.
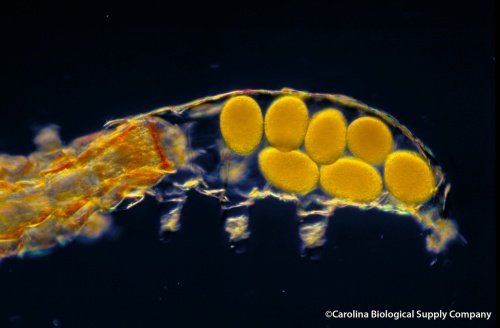
A clutch of seven eggs is left in the empty exoskeleton while the female leaves. Photo by Carolina Biological Supply Company.**
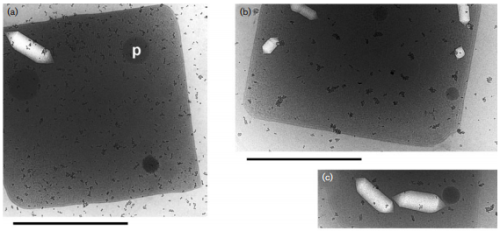
When the habitat of the common water bear gets dry, it enters in a state called cryptobiosis, in which the body shrinks and the metabolism stops. Under this state, known as tun, it can withstand high doses of radiation and both high and zero air pressure, surviving even in the environment of outer space. It is not invincible, however. Radiation in doses above 1000 Gy may not always kill them, but always let them sterile, which is, evolutionary, basically the same thing.
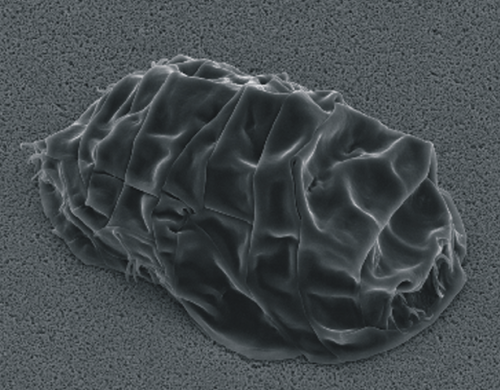
SEM image of the common water bear in the tun state. Photo extracted from Schokraie et al. (2012).*
Nevertheless, the American cockroach is just an amateur regarding survival when compared to the common water bear.
– – –
References:
Horikawa, D. D.; Sakashita, T.; Katagiri, C.; et al. (2009) Radiation tolerance in the tardigrade Milnesium tardigradum. Internatonal Journal of Radiation Biology, 86(12): 843–848. http://dx.doi.org/10.1080/09553000600972956
Schokraie E, Warnken U, Hotz-Wagenblatt A, Grohme MA, Hengherr S, et al. (2012) Comparative proteome analysis of Milnesium tardigradum in early embryonic state versus adults in active and anhydrobiotic state. PLoS ONE 7(9): e45682. https://dx.doi.org/10.1371/journal.pone.0045682
Suzuki, A. C. (2003) Life history of Milnesium tardigradum Doyère (Tardigrada) under a rearing environment. Zoological Science 20(1): 49–57. https://doi.org/10.2108/zsj.20.49
– – –
*
This work is licensed under a Creative Commons Attribution 2.5 Generic License.
**
This work is licensed under a Creative Commons Attribution-NonCommerical-NoDerivs 2.0 Generic License.